|
|

|
||||||||||
|
|||||||||
|
What's New: Recently added: article on Feeding Tropical Fish. |
|||||||||
|
|||||||||
|
|
|||||||||
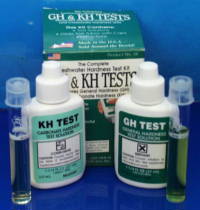
Hardness... and how to change it
The hardness of water is related to the dissolved minerals in contains. The total hardness is usually regarded as consisting of two components: general hardness (GH) and carbonate hardness (KH). General hardness is caused by divalent metal ions, primarily calcium and magnesium. Carbonate hardness is due to carbonate/bicarbonate ions, and represents the main 'buffering capacity' of the water, i.e. its ability to resist pH changes. KH and pH are therefore inter-related: if KH is high, the pH will be very stable (and difficult to alter), if KH is low (e.g. less than 3 degrees KH) then the pH will be less stable.
Carbonate hardness is sometimes referred to as 'temporary' hardness, because it can be removed by boiling,
which precipitates the carbonates. The remaining hardness contributed by other salts of calcium and magnesium is
then referred to as permanent hardness, or non-carbonate hardness. Increasing hardness is normally fairly easy. Adding decor to the tank which will gradually leach hardening salts (such as limestone rock) is one way. Hardening materials such as coral gravel could also be added to a canister filter. There are also commercially available hardening salts - these are often used for tanks containing cichlids from the African rift lakes, which are hard and alkaline.
Decreasing hardness can be done in two main ways: dilution with softer water, or adsorption of
hardening ions. Some people use reverse osmosis (RO), distilled, deionised (DI) or rainwater to dilute their
tapwater to a hardness suitable for their fish. Note that hardness has a fairly straight-forward relationship
with dilution. For example, if your tapwater has a GH of 10 and you use half tapwater and half pure water,
the GH will be 5.
Note that normal table salt (sodium chloride), does not increase hardness. Although it will increase the overall mineral content of the water (and its 'ionic strength'), it is not a hardening salt, like the salts of calcium and magnesium, so will not be measured by a normal GH kit.
Some related terms and definitions:
Alkalinity - This term, used more in marine fishkeeping, historically referred to the buffering capacity of the carbonate-bicarbonate system, but is often used interchangeably with acid neutralising capacity. It is measured in milli-equivalents, or meq. For freshwater aquaria (or most natural freshwaters), the KH value is generally sufficient. Acid Neutralising Capacity - This term is perhaps a more accurate term than alkalinity, because as it suggests, it measures the ability of the water to neutralise acid and measures all the chemical constituents present which can do so. Total Dissolved Solids (TDS) - This term refers to a measure of all the inorganic solids dissolved in the water, so it will measure ions which contribute to hardness, like calcium, but also those that do not, like sodium. It is therefore a better reflection of the total mineral content of the water than hardness measurements. It may be useful for those wishing to accurately reproduce very soft water with a low mineral content - for most purposes, GH and KH provide sufficient information. TDS and other similar measurements can be determined in a number of ways. It may derived from the related conductivity measurement (the method most accessible to aquarists), or determined by the 'dry weight' of solids left after evaporating the water, under laboratory conditions. However, a more satisfactory method is to determine the sum of the major ions in the water. Conductivity (or conductance), is a similar measurement, determined by an electronic meter in micro/milli-Siemens, or alternatively the instrument may be calibrated to give a reading in PPM (parts-per-million). Conductivity increases with increasing ion content, so in most situations it gives a close approximation of the TDS assessed by other means. This measurement is affected by temperature, so is normally standardised to 25oC. Salinity is a term most often associated with marine fishkeeping, although it is also the correct term to refer to the total ion content of freshwater. However, the salinity of freshwater is too low to be determined by aquarium hydrometers, which measure specific gravity, which can then be converted.
[Home] [Article Library] [Fish Index] [Tank Setups] [Forum] [Site Map] |
|
|
| The Tropical Tank Copyright © 2000-2022 Sean Evans | This website was last updated on 20th November 2022 |